Regulatory Trends in Cosmetics: A 12-18 Month Outlook

This blog was originally posted on 11th November, 2025. Further regulatory developments may have occurred after publication. To keep up-to-date with the latest compliance news, sign up to our newsletter.
A 12-18 Month Outlook for Regulatory Trends in Cosmetics
Companies need to stay abreast of changing cosmetics requirements to ensure product compliance & consumer safety, mitigate risk, and send their products to market quickly and efficiently. Of particular interest are US and EU requirements.
The US MoCRA charges the US FDA to make rules on talc testing (the Notice of Proposed Rulemaking was published on 27 Dec. 2024), fragrance allergens, and GMPs; however, the current US government shutdown will likely affect the timing of the rulemaking processes.
In the EU, a proposal to amend Regulations (EC) No 1272/2008 and (EC) No 1223/2009 to simplify requirements pertaining to labeling and CMR (carcinogenic, mutagenic, or reprotoxic) classification, respectively, was drafted in July of 2025. Proposed compliance deadlines begin in June of 2026.
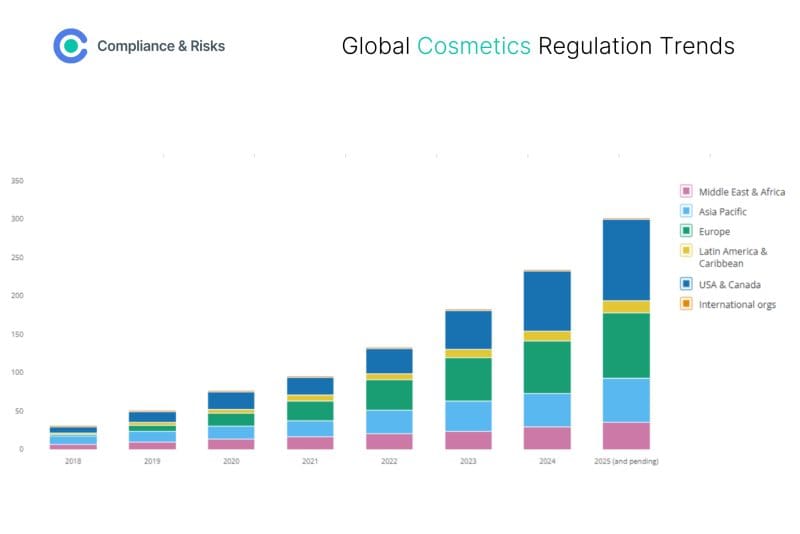
(Source: C2P by Compliance & Risks)
The figure above shows a 900% increase in regulations for cosmetics since 2018, with 300 regulations in place by 2025 (and pending!)
Hot Topics in Cosmetics
Listed below are the key regulations that will be impacting your business over the next 12-18 months:
Safety in the Limelight
A critical–and growing–issue impacting consumer health and safety is that of counterfeit cosmetics. Fake cosmetics often are not tested and therefore may contain ingredients that may cause allergic reactions, chemical burns, infections, or irritation. These products may be more likely to contain carcinogens, mutagens, or reprotoxins, as well.
A representative at the OECD stated: “Counterfeiters do not care about consumer health or safety – full stop. Their sole objective is profit. Any measure ensuring product safety usually involves additional costs that cut their margins. Since these operators already operate outside the law by violating intellectual property rights, they disregard health and safety regulations similarly. There are no standards, no quality controls, and no accountability.”
Quote provided by Personal Care Insights.
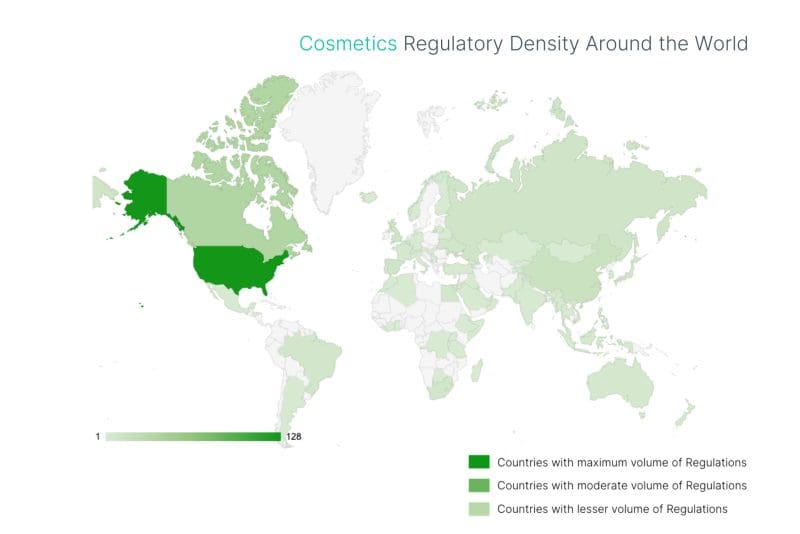
(Source: C2P by Compliance & Risks)
Top Regulatory Trends in Cosmetics
Listed below are the key regulations that will be impacting your business over the next 12-18 months:
- EU: Cosmetic Products Regulation (EC) 1223/2009
- EU: Glossary of Common Ingredient Names in the Use of Labelling Cosmetic Products, Decision (EU) 2025/1175
- Japan: Certification of Excellent Environmental Design for Household Cosmetic Plastic Containers, Notice No. 113, 2025
- China: Cosmetics Supervision and Administration Regulation (CSAR)
- China: Instructions for Use of Consumer Products – General Labelling for Cosmetics, GB 5296.3-2008
- USA: Modernization of Cosmetics Regulation Act (MoCRA), 2022
- USA: Federal Food, Drug & Cosmetic Act, 2009
- Hawaii (USA): Prohibition of Plastics Microbeads; Personal Care Products; Non-prescription Drugs, Revised Statutes §321-30.5, Act 156, 2022
- Egypt: Required Documentation for the Manufacture and Import of Cosmetics, Regulatory Guide, 2025
- Panama: Ban of Trimethylbenzoyl Diphenylphosine Oxide and Dimethyltolylamine in Cosmetics, Resolution No. 218, 2025
- UK: Cosmetic Products (Restriction of Chemical Substances) Regulations, SI No. 455/2024
- Canada: Cosmetic Regulations CRC. 869, 2021
- Canada: Cosmetic Ingredient Hotlist, 2024
- ASEAN: Association of Southeast Asian Nations Cosmetic Directive
Stay Up To Date With Global Regulatory Trends In Cosmetics
Book Time With Our Team
Learn how C2P can help you stay ahead of global Product Safety regulatory changes and achieve uninterrupted market access.