We have the ability to record information inside C2P, directly alongside relevant legislation and our related action plans.
Sebastien Marcel
Global Environment Manager, Markem-Imaje

technology
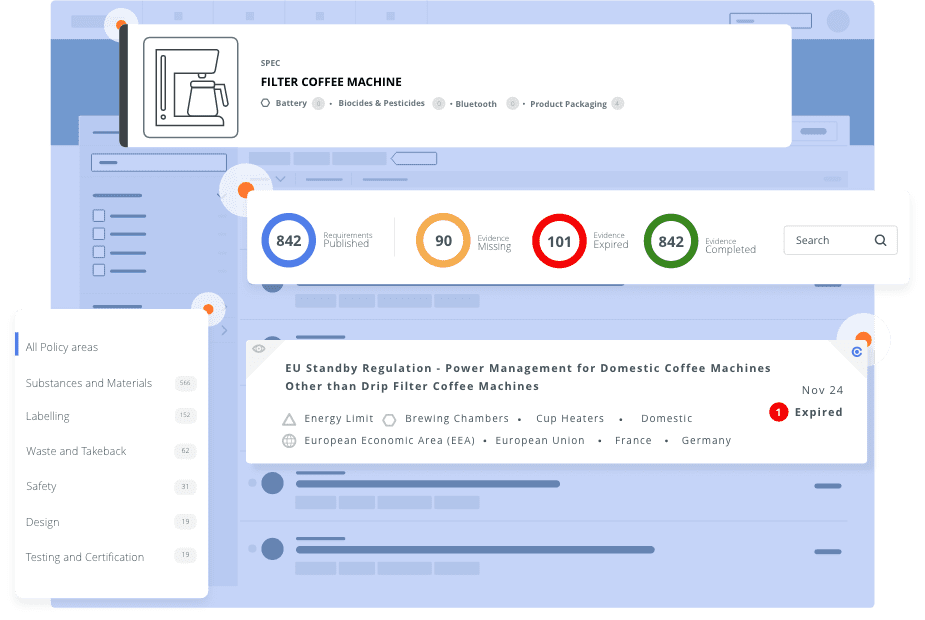
Can product ‘X’ be shipped to market ‘Y’? A simple question with a sometimes complex answer as the information is often spread throughout several departments and systems with some information that is out of date or even missing. Selling and shipping in multiple markets around the world calls for an easily accessible solution to store and manage evidence of compliance. It calls for C2P Evidence Collection Software.
C2P is here to help you join all the compliance dots in an end-to-end market access and product compliance solution. Link Compliance Evidence to Product Requirements and all informing Regulations, Standards, Customer and other Requirements. And with automated alerts you can manage it all by exception and monitor ever-changing compliance obligations.

The need for timely, comprehensive and accurate regulatory information impacting your products is greater than ever before. Stay ahead of these changes in Regulations and Standards with C2P.
Let Compliance and Risks help you unlock market access.
Our demo team can show you how this would work for you and your team. Get in touch now and see C2P in detail.