Regulatory Trends in Medical Devices 2024: A 12-18 Month Outlook
A 12-18 month outlook for regulatory trends in medical devices
Traditionally, tightly controlled due to the impact they may have on human life, Medical Devices are witnessing a further expansion in regulation with recent developments such as the overhaul of the medical device regime in the EU, a focus on medical device traceability, concerns about cybersecurity and more.
These changes, in tandem with concerns regarding restriction of chemicals substances in products and other regulations pertinent to electronics, in general, mean that manufacturers of medical devices need to continuously monitor and assess regulatory requirements to ensure compliance.
Figure 1 below shows this growth has reached unprecedented levels with year on year increase in Regulations for medical device manufacturers since 2015.
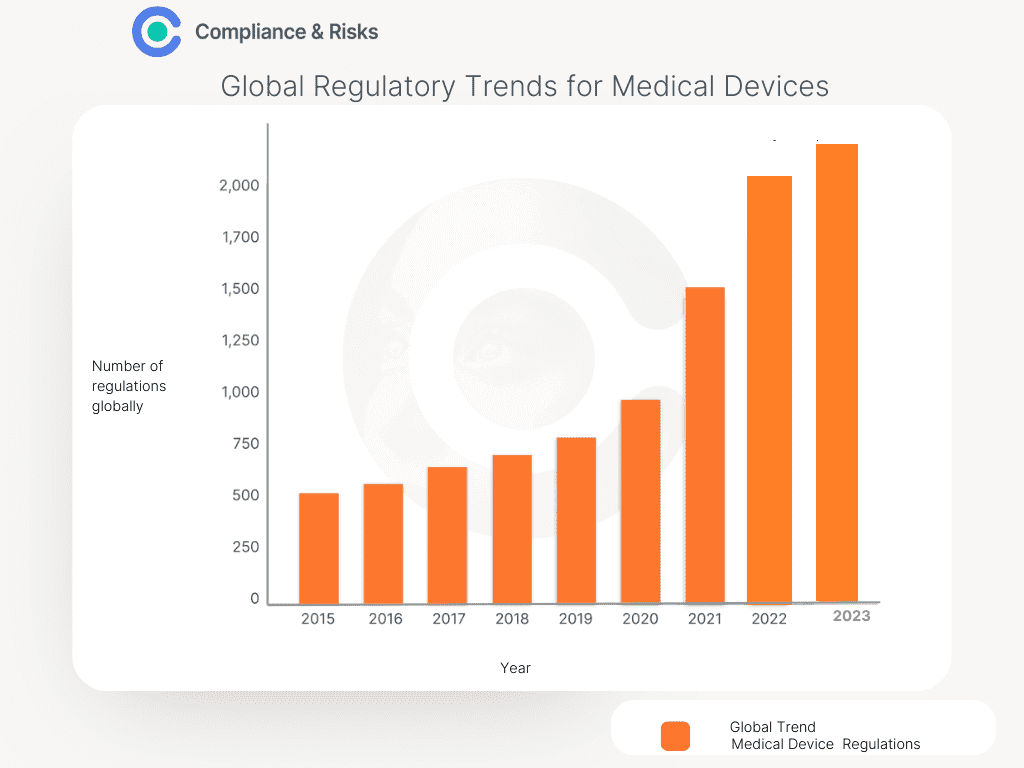
(Source: C2P by Compliance & Risks)
Key Areas of Concern & Hot Topics
Here’s a bird’s-eye view of key regulatory trends and issues you need to be aware of to get your product on the market and keep it there:
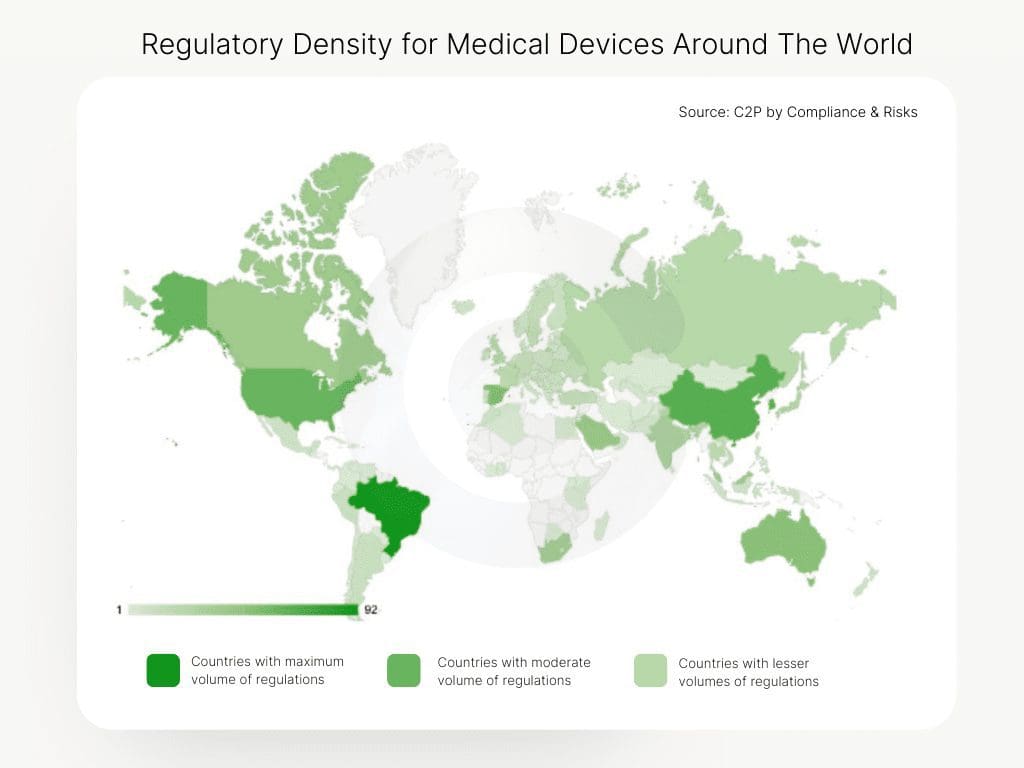
(Source: C2P by Compliance & Risks)
Top Trending Regulations
Listed below are the key regulations that will be impacting your business over the next 12-18 months:
- USA: Quality System for Medical Device Manufacturing, 21 CFR Part 820 – Amendment – (on quality system regulation), Final Rule, 89 FR 7496, February 2024
- EU: In Vitro Diagnostic Medical Devices, Regulation (EU) 2017/746 and Others – Proposed Amendment – (on extended transition timelines, etc.) Draft Regulation, January 2024
- UK: Implementation of Medical Devices Future Regime, Guidance Document, January 2024
- Hong Kong (China): Artificial Intelligence Medical Devices (AI-MD), Guidance Document, TR-008, January 2024
- California (USA): Prohibition of Di-(2-ethylhexyl) phthalate (DEHP) in Medical Devices, Assembly Bill 2300, 2024
- Norway: REACH Enforcement, Regulation No. 516, 2008 – Amendment – (on implementation of Regulation (EU) 2023/2482) Regulation No. 191, 2024
- Brazil: Risk Classification, Notification, Registration and Labelling Requirements for In Vitro Medical Devices, Resolution No. 830/2023
- EU: Medical Devices, Regulation (EU) 2017/745 and Others – Amendment – (on transitional provisions for certain medical devices and in vitro diagnostic medical devices) Regulation (EU) 2023/607
- EU: Q&A on Practical Aspects Related to the Implementation of Regulation (EU) 2023/607, Guidance Document, Revised, July 2023
Stay Up To Date With Global Regulatory Trends In Medical Devices
Book Time With Our Team
Learn how C2P can help you stay ahead of global medical device regulatory changes and achieve uninterrupted market access.








