
C2P Technology Spotlight

Welcome to our C2P Technology Spotlight!
We are excited to bring you some key feature enhancements to C2P that will help you to simplify your regulatory compliance process.
Workflow: From Alert to Compliance
Our SME users have challenged us to provide better workflow tools to manage a regulation right through from receiving an alert from C2P, to creating requirements applicable to their products, and managing the related evidence. This is one of many steps we have planned to seamlessly connect regulations and requirements, and we are off to a great start.
C2P users, who are monitoring regulations in order to keep their requirements up to date, can now:
- See at a glance if a new or updated regulation/standard has existing requirements linked
- View the list of requirements related to the regulation/standard, so that you can assess at a glance if a requirement needs to be added or changed
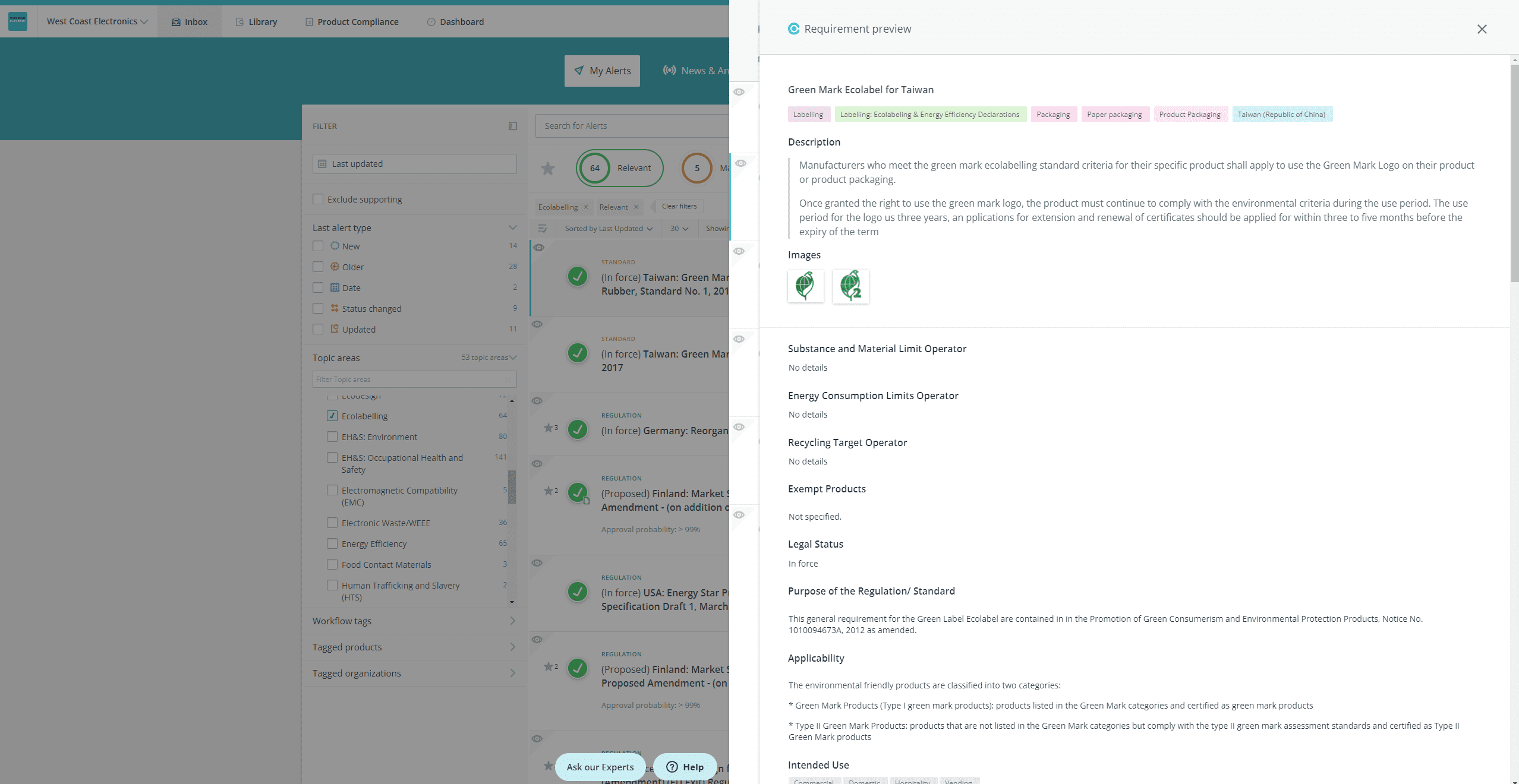
- Open the requirement preview panel so that you can see the requirement details, and, from the inbox:
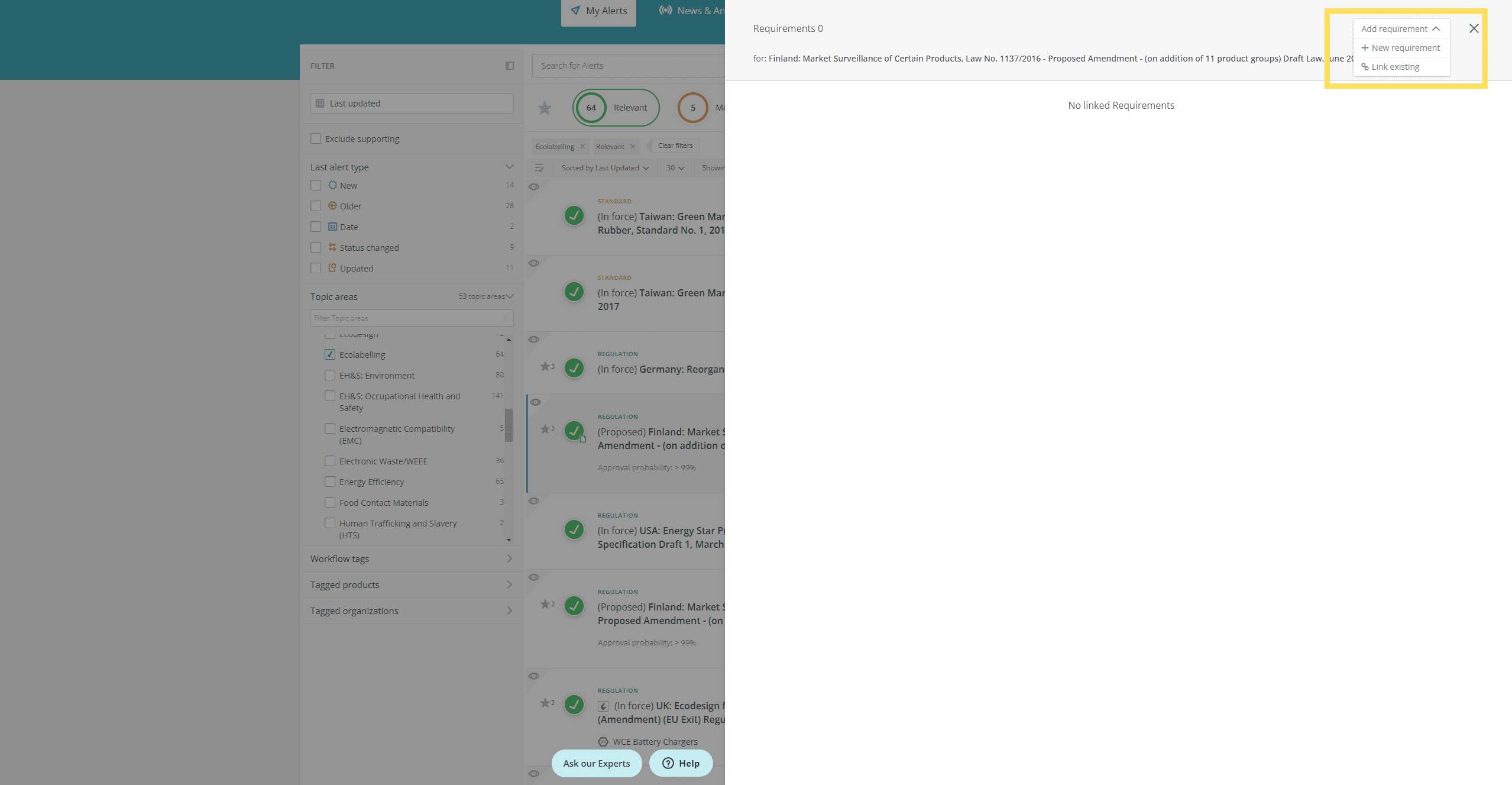
- Edit a requirement
- Create a new requirement
- Link an existing requirement
See at a glance if a new or updated regulation/standard has existing requirements linked
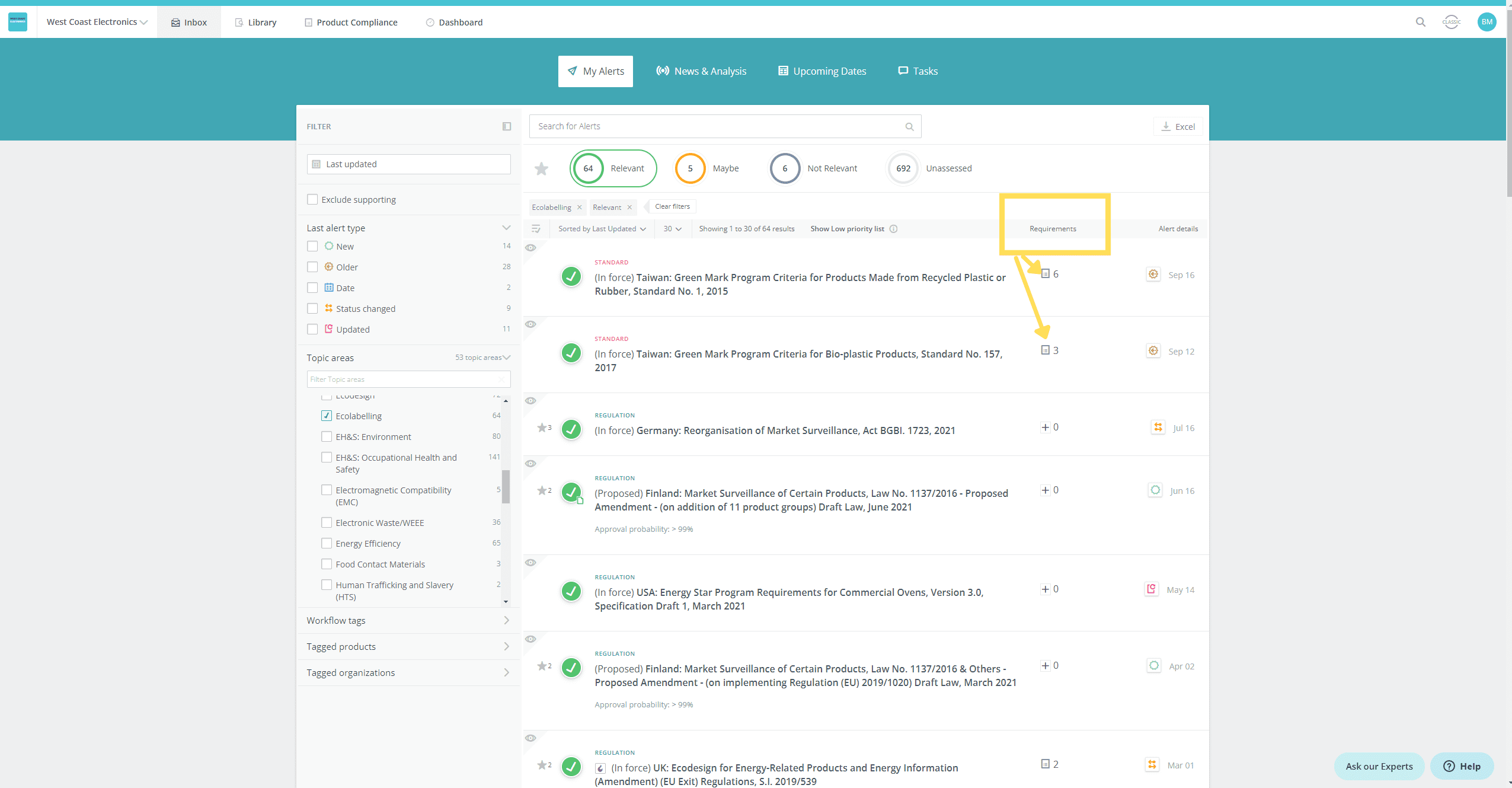
View the list of requirements related to the regulation/standard
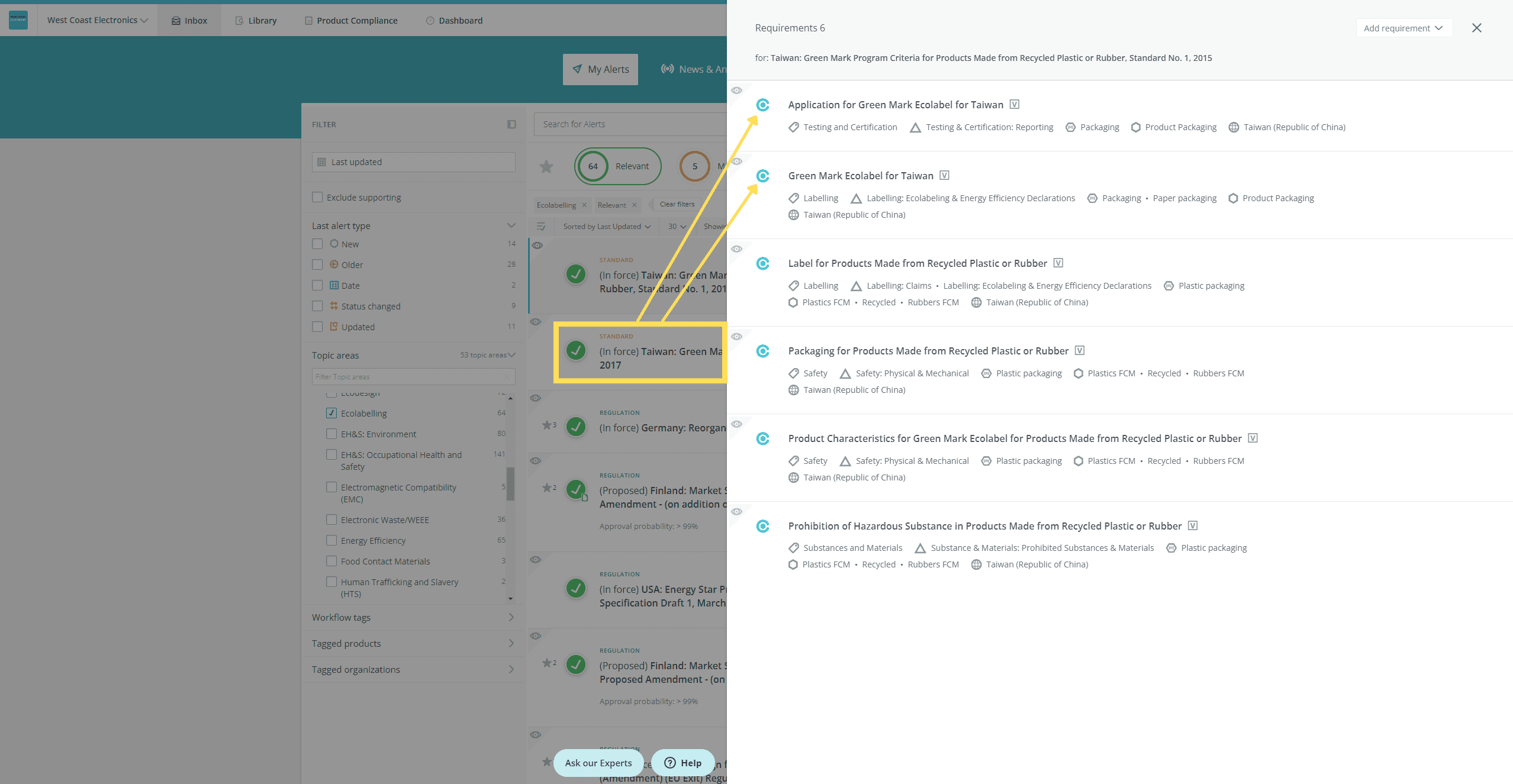
Drill down to a specific requirement to see the requirement details
Create, edit and link requirements from the inbox
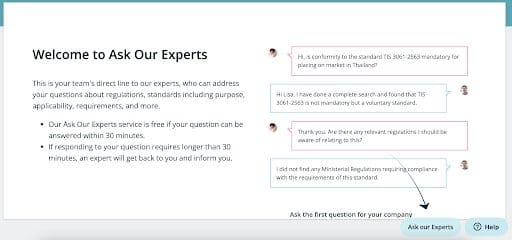
‘Ask Our Experts’ Made More Accessible
We recently released My Company Ask Our Experts in C2P, to allow our users to access the full collection of questions ever asked by their colleagues. Since then, we have received many good suggestions. As a result we’ve added a number of key features:
- For those who have never used ‘Ask Our Experts’ before, we have a new introductory page to show you how it works
- You can now preview the regulation, standard or requirement connected to your Ask Our Experts question from My Company Ask our Experts
- You can also export a report of all of your company’s ‘Ask Our Experts’ to excel
New Content: Medical Devices
C2P now incorporates new global Medical Device coverage, in what is already the world’s most extensive product compliance library. Subscribers can now use C2P to monitor the latest proposed, enacted and amended regulations and mandatory standards worldwide.
In-force regulations and mandatory standards for 68 major regions with global monitoring in 226 geographies means Medical Device clients can now avail of comprehensive coverage & monitoring of the regulations and standards that impact their products & markets.
Find out more about our medical devices coverage.
You can email our support team at support@complianceandrisks.com if you have any questions on the new features listed above.
Not yet a C2P user but want to find out more? Book a demo today!
Market Insights straight to your inbox
Join 30,000+ product compliance & market access experts around the world








